Iowa City Ambulatory Surgical Center (Iowa City ASC) is the first center in Iowa to offer Inspire therapy. Inspire therapy is a new, clinically proven treatment option, approved by the FDA, for people with Obstructive Sleeve Apnea (OSA). Inspire Therapy is specifically designed for people with moderate to severe OSA who are unable to use Continuous Positive Airway Pressure (CPAP).
Among its many advantages, Inspire therapy is fully implanted during an outpatient procedure, does not require a mask or oral appliance, works with your body’s natural anatomy, and is simple and easy to use. Our doctors and staff are equipped to counsel and help you determine if you would be a good candidate for this treatment.
How does inspire work?
Inspire is a breakthrough therapy for people with Obstructive Sleep Apnea (OSA). While CPAP and oral appliances work from “the outside in” during sleep to prevent your tongue and other soft tissues from relaxing and obstructing your airway, Inspire therapy works from “the inside out.”
While you sleep, Inspire monitors every breath you take. Based on your unique breathing patterns, the system delivers mild stimulation to key airway muscles, which keeps the airway open during sleep.
Inspire therapy is a fully implanted system that is controlled with a handheld Inspire sleep remote. There are three components that are implanted:
- A small generator
- A breathing sensor lead
- A stimulation lead
Using the small handheld sleep remote, you simply turn Inspire therapy on at night before bed and turn it off in the morning when you wake up.
Am I A Candidate?
You might be a candidate for Inspire therapy if:
- you have moderate to severe Obstructive Sleep Apnea (AHI of 20 to 65)
- you are unable to use or get consistent benefit from CPAP
- you are not significantly overweight
- you are over the age of 22
An Inspire therapy-trained doctor will evaluate your overall health status and perform a physical examination of your airway to determine if Inspire therapy might be a suitable CPAP alternative for you. If you are determined to be a good candidate for Inspire therapy, your doctor will work with your insurance company to gain insurance coverage on your behalf. This process can take a few weeks to a few months. Surgery will generally take place 1 to 4 weeks following insurance approval.
Then you are ready to enjoy a quiet, comfortable, and restful night’s sleep!

What can I expect during/after The procedure?
The Inspire procedures performed at Iowa City Ambulatory Surgical Center are performed in an outpatient setting under general anesthesia. The Inspire therapy system is fully implanted under the skin of the neck and chest.
You may have some discomfort or swelling at the small incision sites for a few days. This is generally managed with ibuprofen or acetaminophen. Most people return to their regular diet and non-strenuous activities within a few days.
Three to four weeks following surgery you will have an appointment with your doctor. The doctor will activate the implant for the first time, establish your unique therapy settings then show you how to use the hand sleep remote.
One month after the device is activated, physicians optimize therapy settings for a patient during a routine sleep study. It’s also common to see your doctor once a year for a check-up.
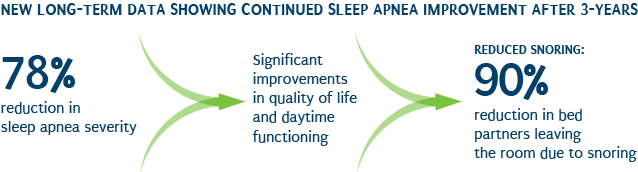
Proven results
The Star (Stimulation Therapy for Apnea Reduction) trial was a clinical trial designed to evaluate the safety and effectiveness of Inspire Therapy. Results were published in the January 9, 2014 edition of the New England Journal of Medicine. Patients who participated in the STAR trial experienced significant reductions in sleep apnea events and significant improvements in quality of life measures, including:
- 68 percent reduction in episodes of sleep apnea
- 70 percent reduction in oxygen desaturation events (ODI)
- 85 percent of bed partners reported no snoring or soft snoring for partners using Inspire therapy
- Significant improvement in daytime functioning as measured by Epworth Sleepiness Scale (ESS) and Functional Outcomes of Sleep Questionnaire (FOSQ)
Inspire therapy is clinically proven and FDA approved.












